CBSE Class 12 Chemistry Question Paper 2024 (Set 2 - 56/2/2) with Answer Key
CBSE Class 12 Chemistry Question Paper 2024 PDF (Set 2 - 56/2/2) is available for download here. CBSE conducted the Chemistry exam on February 27, 2024, from 10:30 AM to 1:30 PM. The total marks for the theory paper are 70. The question paper contains 20% MCQ-based questions, 40% competency-based questions, and 40% short and long answer type questions.
CBSE Class 12 Chemistry Question Paper 2024 (Set 2 - 56/2/2) with Answer Key
| CBSE Class 12 2024 Chemistry Question Paper with Answer Key | Check Solution |
CBSE Class 12 Chemistry Question Paper 2024 with Solution

Section A
The product of oxidation of \( SO_3^{2-} \) with \( MnO_4^{-} \) in acidic medium is:
View Solution
In an acidic medium, permanganate ion \( MnO_4^{-} \) acts as a strong oxidizing agent. It oxidizes sulfite \( SO_3^{2-} \) to sulfate \( SO_4^{2-} \). The balanced reaction is:
\[ SO_3^{2-} + MnO_4^{-} + H^+ \rightarrow SO_4^{2-} + Mn^{2+} + H_2O \]
This oxidation occurs due to the ability of \( MnO_4^{-} \) to gain electrons and reduce to \( Mn^{2+} \), making sulfate the stable oxidation product of sulfite. Quick Tip: In acidic oxidation reactions, permanganate ion is often used as a strong oxidizing agent, converting sulfite to sulfate.
The coordination number of Co in \( [Co(en)_3]^{3+} \) is:
View Solution
In coordination chemistry, the coordination number is determined by the number of ligand attachment points to the central metal atom. Ethylenediamine (\( en \)) is a bidentate ligand, meaning each molecule binds to the central cobalt (\( Co^{3+} \)) at two sites. Since three \( en \) ligands are present, the total coordination number is:
\[ 3 \times 2 = 6 \]
This results in an octahedral geometry around the cobalt center. Quick Tip: A bidentate ligand contributes two binding sites, effectively increasing the coordination number.
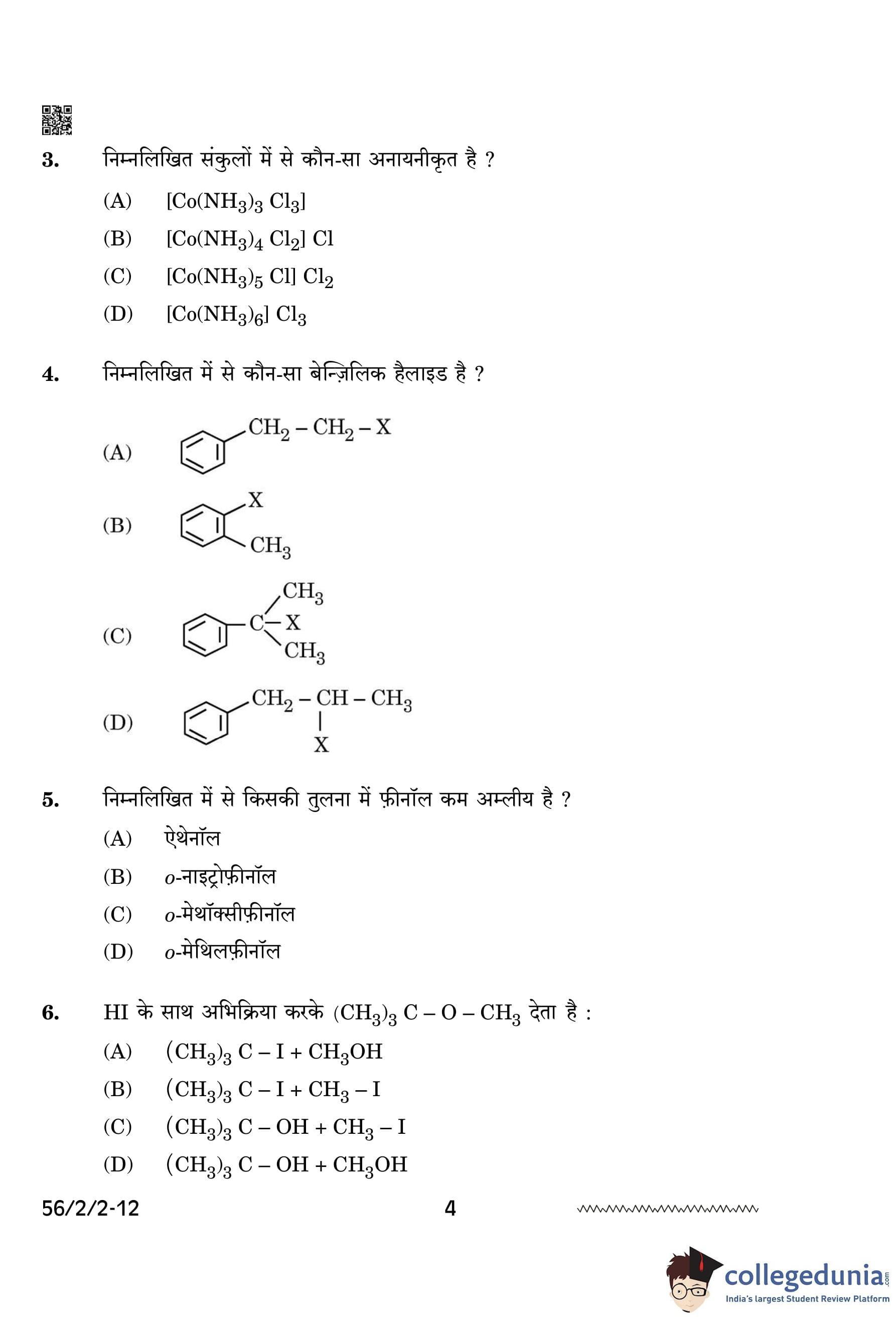
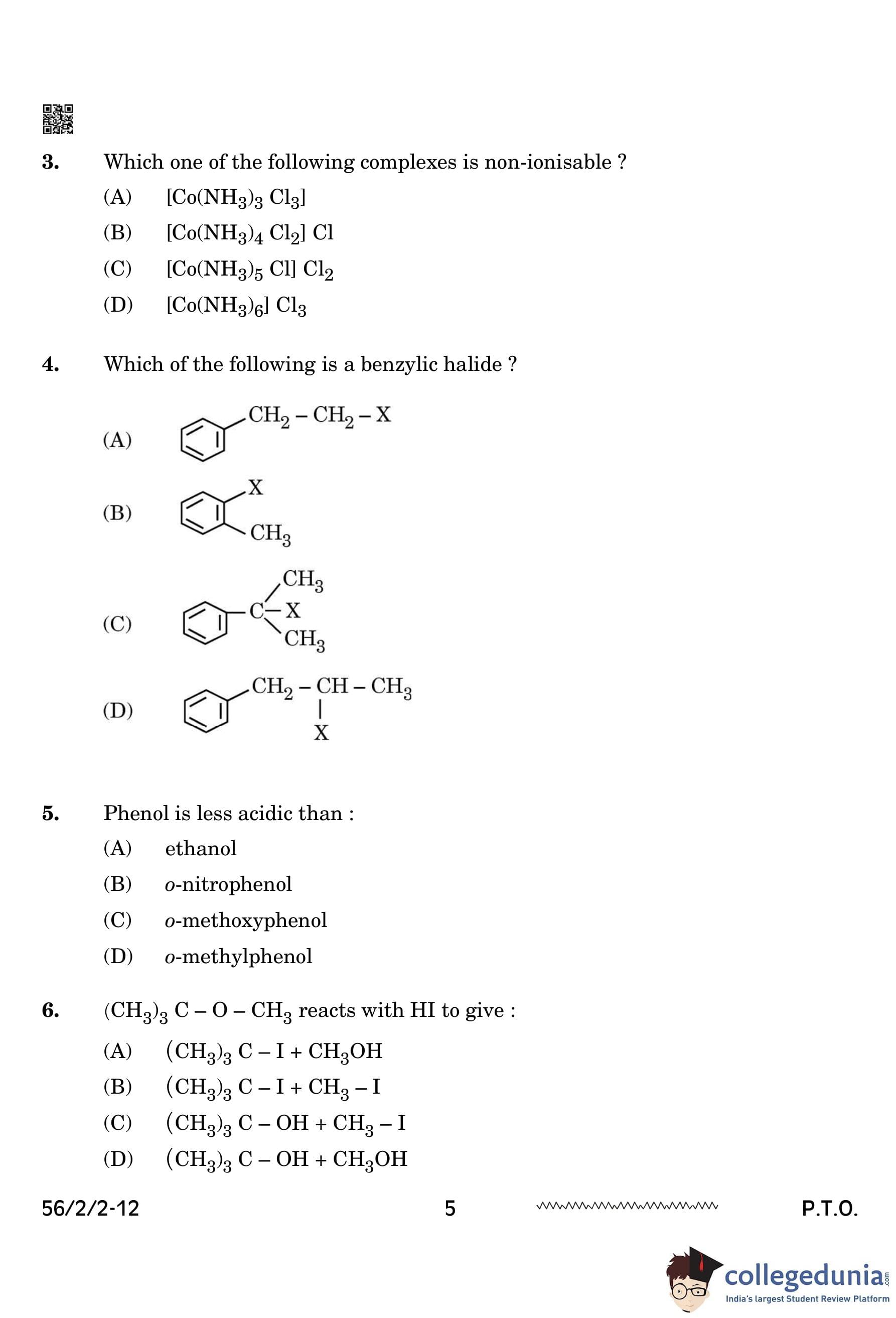
Which one of the following complexes is non-ionisable?
View Solution
A complex is considered non-ionizable when all its ligands are directly bonded to the central metal ion with no counter-ions available for dissociation in solution. In \( [Co(NH_3)_3Cl_3] \), all three chloride (\( Cl^- \)) ligands are directly coordinated to cobalt, meaning it does not dissociate in water. The other options have external chloride ions that ionize in solution. Quick Tip: Non-ionizable coordination compounds have all anionic ligands bonded to the central metal with no free counter-ions.
Which of the following is a benzylic halide?
View Solution
A benzylic halide contains a halogen (\( X \)) directly attached to a benzylic carbon (a carbon adjacent to a benzene ring). In option (C), the halogen is directly connected to the carbon adjacent to the benzene ring, making it a benzylic halide. Quick Tip: Benzylic halides exhibit high reactivity in SN1, SN2, E1, and E2 reactions because of the resonance stabilization of the benzylic carbocation and radical intermediates. The benzylic position allows for rapid nucleophilic substitution and elimination due to its enhanced stability.
Phenol is less acidic than:
View Solution
Phenol is less acidic than o-nitrophenol because the nitro group (\( -NO_2 \)) is an electron-withdrawing group, stabilizing the phenoxide ion through resonance and inductive effects. This increases the acidity compared to plain phenol. Quick Tip: Electron-withdrawing groups enhance acidity, whereas electron-donating groups reduce it.
\( (CH_3)_3 C - O - CH_3 \) reacts with HI to give:
View Solution
HI cleaves the ether bond through an SN1 mechanism, forming tertiary iodide and methanol:
\[ (CH_3)_3 C - O - CH_3 + HI \rightarrow (CH_3)_3 C - I + CH_3OH \] Quick Tip: Tertiary ethers undergo cleavage via an SN1 pathway in acidic conditions. The protonation of the ether oxygen makes the leaving group more stable, facilitating the formation of a carbocation.
Aspirin is formed by the acetylation of:
View Solution
Aspirin, also known as acetylsalicylic acid, is synthesized by the acetylation of salicylic acid using acetic anhydride. This reaction results in the formation of an ester bond between the hydroxyl group of salicylic acid and the acetyl group. Quick Tip: Aspirin is a widely used pain-relieving drug synthesized by esterification. It is prepared from salicylic acid by acetylation using acetic anhydride, increasing its stability and reducing gastric irritation.
An azeotropic solution of two liquids has a boiling point higher than either of them when it:
View Solution
A solution exhibits a higher boiling azeotrope when it shows negative deviation from Raoult’s law. This happens when intermolecular forces between solute and solvent are stronger than in the pure components, leading to decreased vapor pressure and increased boiling point. Quick Tip: Negative deviation from Raoult’s law occurs when solute-solvent interactions are stronger than those in pure components, lowering vapor pressure and raising the boiling point significantly.
During electrolysis of aqueous CuCl\(_2\):
View Solution
During the electrolysis of aqueous \( CuCl_2 \), copper ions (\( Cu^{2+} \)) gain electrons at the cathode and get deposited as solid copper:
\[ Cu^{2+} + 2e^- \rightarrow Cu (s) \]
At the anode, chloride ions (\( Cl^- \)) lose electrons and form chlorine gas:
\[ 2Cl^- \rightarrow Cl_2 (g) + 2e^- \] Quick Tip: In electrolysis, cations migrate to the cathode to gain electrons (reduction), while anions migrate to the anode to lose electrons (oxidation), forming respective elements.
Half-life period of a first-order reaction is 1386 seconds. The rate constant (\( k \)) of the reaction is:
View Solution
For a first-order reaction, the half-life (\( t_{1/2} \)) is related to the rate constant (\( k \)) by:
\[ t_{1/2} = \frac{0.693}{k} \]
Substituting \( t_{1/2} = 1386 \) s:
\[ k = \frac{0.693}{1386} = 0.5 \times 10^{-3} \, s^{-1} \] Quick Tip: First-order reactions have a constant half-life, and their rate constant can be found using \( k = 0.693/t_{1/2} \), which remains independent of initial concentration.
The relative lowering of vapor pressure of an aqueous solution containing non-volatile solute is 0.0225. The mole fraction of the non-volatile solute is:
View Solution
The relative lowering of vapor pressure is given by Raoult’s Law:
\[ \frac{\Delta P}{P_0} = X_{solute} \]
Since the given relative lowering of vapor pressure is 0.0225, the mole fraction of the solute is also 0.0225. Quick Tip: Raoult’s Law states that relative lowering of vapor pressure equals the mole fraction of solute in an ideal dilute solution, applicable for non-volatile solutes.
The rate of the reaction \( 2A + B_2 \rightarrow 2AB \) is given by:
\[ Rate = k[A]^2[B_2] \]
The value of the rate constant (\( k \)) can be increased by:
View Solution
The rate constant (\( k \)) depends only on temperature and not on reactant concentration. According to the Arrhenius equation:
\[ k = A e^{-E_a/RT} \]
where \( E_a \) is the activation energy. Increasing temperature decreases the exponential factor, leading to an increase in \( k \). Quick Tip: While reaction rate depends on concentration, the rate constant (\( k \)) changes only with temperature, governed by the Arrhenius equation and activation energy.
Assertion (A): Aromatic primary amines can be prepared by Gabriel phthalimide synthesis.
Reason (R): Alkyl halide undergoes nucleophilic substitution with anion formed by phthalimide.
View Solution
The Gabriel phthalimide synthesis is used to prepare only aliphatic primary amines, not aromatic amines. This makes the assertion false. However, the reason correctly describes how alkyl halides react with the phthalimide anion to form amines, making it true. Quick Tip: Gabriel phthalimide synthesis is only suitable for aliphatic amines because aryl halides do not undergo nucleophilic substitution under normal conditions.
Assertion (A): Uracil base is present in RNA.
Reason (R): RNA undergoes self-replication.
View Solution
Uracil is indeed present in RNA, replacing thymine found in DNA, making the assertion true. However, RNA does not undergo self-replication like DNA; it requires enzymes such as RNA polymerase to be copied, making the reason false. Quick Tip: RNA contains uracil instead of thymine, and unlike DNA, it requires external enzymes like RNA polymerase for replication.
Assertion (A): Diazonium salts of aliphatic amines are more stable than those of aromatic amines.
Reason (R): Diazonium salts of aromatic amines show resonance.
View Solution
Aromatic diazonium salts are actually more stable than aliphatic diazonium salts due to resonance stabilization. The assertion is therefore false. However, the reason is true because aromatic diazonium salts can delocalize the positive charge via resonance, increasing their stability. Quick Tip: Aromatic diazonium salts are more stable than aliphatic ones due to resonance, which stabilizes the positive charge on the nitrogen.
Assertion (A): Acetanilide \(\left( C_6H_5NHCOCH_3 \right)\) is less basic than aniline.
Reason (R): Acetylation of aniline results in a decrease of electron density on nitrogen in acetanilide.
View Solution
Acetanilide is less basic than aniline because the acetyl group withdraws electron density from the nitrogen via resonance, making the nitrogen less available for protonation. This explains why both the assertion and reason are true, and the reason correctly explains the assertion. Quick Tip: The acetyl group in acetanilide withdraws electron density from nitrogen, reducing its availability for protonation, making it less basic than aniline.
Define the following terms:
(a) Essential amino acids
View Solution
Amino acids which cannot be synthesized in the body and must be obtained through diet.
Essential amino acids are required for protein synthesis and metabolic functions but cannot be produced by the human body. They must be consumed through dietary sources such as meat, eggs, dairy, and legumes. Quick Tip: There are nine essential amino acids for humans: histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine.
(a) In the following pair of compounds, which compound undergoes S\(_N1\) reaction faster and why?
View Solution
In an S\(_N1\) reaction, the rate-determining step is the formation of a carbocation. The benzyl carbocation formed from benzyl chloride is highly stabilized by resonance, allowing the reaction to proceed much faster. In contrast, the cyclohexyl carbocation lacks such stabilization, making it less reactive in S\(_N1\) conditions.
Quick Tip: Benzyl carbocations are highly stable due to resonance delocalization, making benzyl chloride undergo S\(_N1\) reactions much faster than alkyl chlorides.
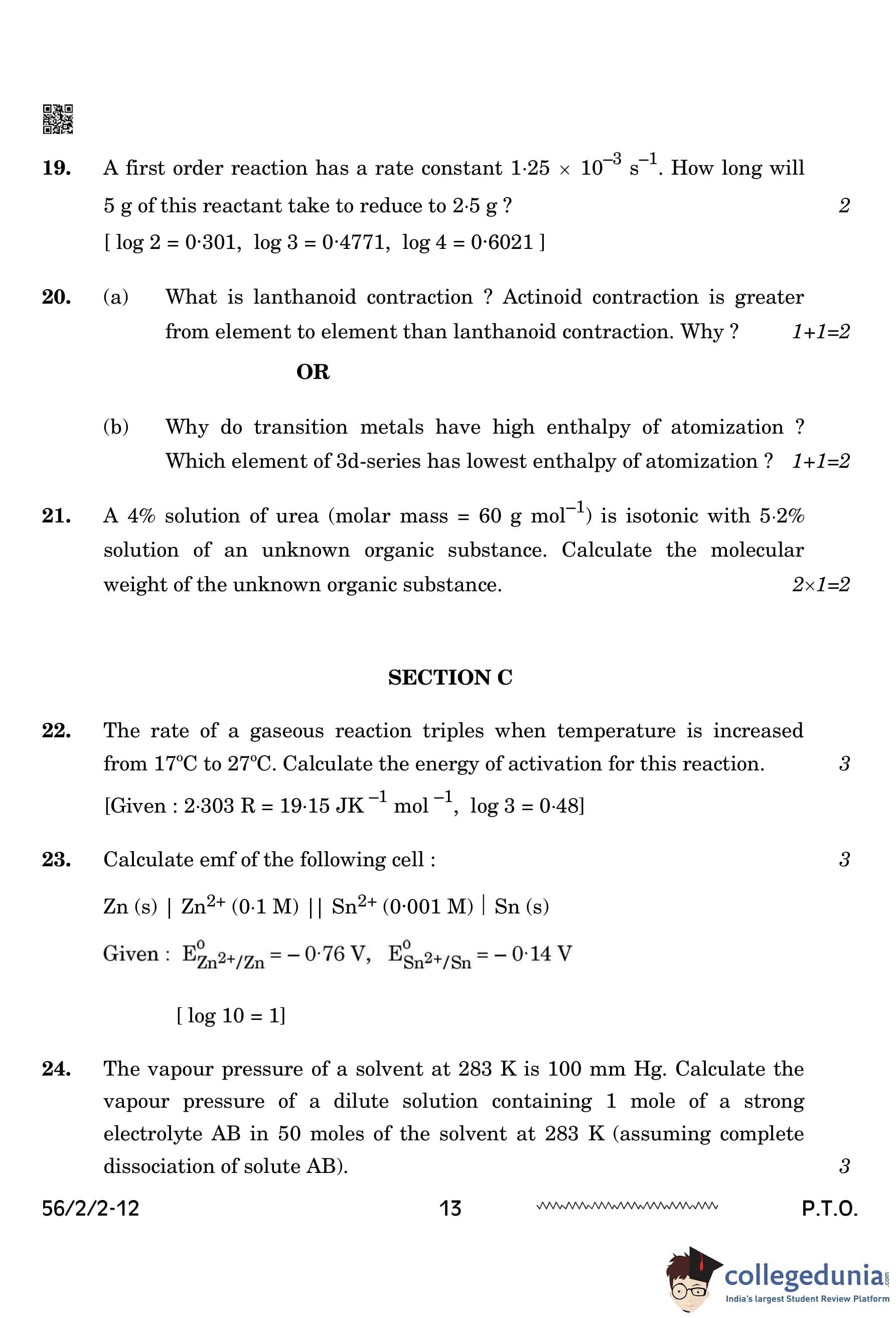
A first-order reaction has a rate constant \(1.25 \times 10^{-3} \, s^{-1}\). How long will 5 g of this reactant take to reduce to 2.5 g?
\[ [ log 2 = 0.301, \quad log 3 = 0.4771, \quad log 4 = 0.6021 ] \]
View Solution
For a first-order reaction, the integrated rate equation is:
\[ k = \frac{2.303}{t} \log \frac{[R]_0}{[R]} \]
Substituting given values:
\[ 1.25 \times 10^{-3} = \frac{2.303}{t} \log \frac{5}{2.5} \]
\[ 1.25 \times 10^{-3} = \frac{2.303}{t} \times \log 2 \]
\[ t = \frac{2.303 \times 0.301}{1.25 \times 10^{-3}} \]
\[ t = 554.5 \, s \quad or \quad 5.54 \times 10^2 \, s \] Quick Tip: For a first-order reaction, the time required for concentration reduction can be calculated using the logarithmic form of the rate equation.
(a) What is lanthanoid contraction? Actinoid contraction is greater from element to element than lanthanoid contraction. Why?
View Solution
Lanthanoid contraction: The steady decrease in atomic or ionic radii with an increase in atomic number due to the poor shielding effect of the 4f subshell.
Actinoid contraction is greater: The 5f subshell has an even poorer shielding effect compared to the 4f subshell, leading to a stronger contraction. Quick Tip: Lanthanoid and actinoid contractions affect the atomic and ionic radii, influencing chemical reactivity and properties of elements in the periodic table.
A 4% solution of urea (molar mass = 60 g mol\(^{-1}\)) is isotonic with 5.2% solution of an unknown organic substance. Calculate the molecular weight of the unknown organic substance.
View Solution
Since the two solutions are isotonic, they have the same osmotic pressure (\(\pi_1 = \pi_2\)). Using the equation:
\[ \frac{w_{urea}}{M_{urea}} = \frac{w_A}{M_A} \]
Substituting the given values:
\[ \frac{4}{60} = \frac{5.2}{M_A} \]
Solving for \( M_A \):
\[ M_A = \frac{5.2 \times 60}{4} = 78.0 \, g \, mol^{-1} \] Quick Tip: For isotonic solutions, the osmotic pressure equation is used to compare the concentration and molecular weight of different solutes.
The rate of a gaseous reaction triples when temperature is increased from 17°C to 27°C. Calculate the energy of activation for this reaction.
\([Given : 2 303 R = 19 15 JK^{-1} mol^{-1}, log 3 = 0 48]\)
View Solution
The Arrhenius equation is:
\[ \log \left( \frac{k_2}{k_1} \right) = \frac{E_a}{2.303R} \left( \frac{1}{T_1} - \frac{1}{T_2} \right) \quad or \quad \frac{E_a}{2.303R} \left[ \frac{T_2 - T_1}{T_1 T_2} \right] \]
Substituting the known values for the rate constants and temperatures into the equation. \(k_2/k_1 = 3\), \(T_1 = 290\,K\), and \(T_2 = 300\,K\):
\[ \log \left( \frac{3k_1}{k_1} \right) = \frac{E_a}{19.15} \left( \frac{1}{290} - \frac{1}{300} \right) \]
we get:
\[ 0.48 = \frac{E_a}{19.15} \left( \frac{10}{290 \times 300} \right) \]
Activation energy, \(E_a\), by multiplying both sides of the equation:
\[ E_a = 0.48 \times 19.15 \times 290 \times 300 \quad \Rightarrow \quad E_a = \frac{0.48 \times 19.15 \times 290 \times 300}{10} \]
Finally, calculate the value of \(E_a\). The result gives the activation energy in J/mol. After performing the calculation, we find:
\[ E_a = 79970 \, J mol^{-1} \quad or \quad E_a = 79.970 \, kJ mol^{-1} \] Quick Tip: - The Arrhenius equation is useful to calculate the energy of activation when the rate constant changes with temperature. - The rate of reaction is highly sensitive to temperature, and small temperature changes can significantly impact the rate.
Calculate emf of the following cell: \[ Zn (s) | Zn^{2+} (0.1 M) || Sn^{2+} (0.001 M) | Sn (s) \]
Given: \[ E^\circ_{Zn^{2+}/Zn} = -0.76 \, V, \quad E^\circ_{Sn^{2+}/Sn} = -0.14 \, V \] \[ [\log 10 = 1] \]
View Solution
The equation for the emf of the cell is: \[ E_{cell} = \left( E^\circ_{c} - E^\circ_{a} \right) - \frac{0.059}{2} \log \frac{[Zn^{2+}]}{[Sn^{2+}]} \]
Substituting the given values: \[ E_{cell} = \left[ (-0.14) - (-0.76) \right] - \frac{0.059}{2} \log \frac{0.1}{0.001} \] \[ E_{cell} = +0.62 - 0.059 \] \[ E_{cell} = (0.62 - 0.059 ) \, V = 0.561 \, V \] Quick Tip: - The Nernst equation helps calculate the emf of electrochemical cells by considering the concentrations of the ions involved. - The formula involves standard reduction potentials and logarithmic terms for concentration ratios.
The vapour pressure of a solvent at 283 K is 100 mm Hg. Calculate the vapour pressure of a dilute solution containing 1 mole of a strong electrolyte AB in 50 moles of the solvent at 283 K (assuming complete dissociation of solute AB).
View Solution
The formula for the relative lowering of vapour pressure is: \[ \frac{p^\circ - p_s}{p^\circ} = i \times \chi \]
Where:
- \( p_0 \) is the vapour pressure of the pure solvent
- \( p_s \) is the vapour pressure of the solution
- \( i \) is the Van't Hoff factor (which represents the number of particles produced from dissociation; for a strong electrolyte AB, \( i = 2 \))
- \( \chi \) is the mole fraction of the solute
For a dilute solution: \[ \frac{100 - p_s}{100} = 2 \times \frac{n_{AB}}{n_{solvent}} \]
Where:
- \( n_{AB} = 1 \) mole (the amount of solute AB)
- \( n_{solvent} = 50 \) moles (the amount of solvent)
Substituting these values in: \[ \frac{100 - p_s}{100} = 2 \times \frac{1}{50} \]
Simplifying: \[ \frac{100 - p_s}{100} = \frac{2}{50} \] \[ 100 - p_s = 4 \] \[ p_s = 96 \, mm Hg \] Quick Tip: - The Van't Hoff factor \( i \) is critical in determining the effect of dissociation on the vapour pressure of a solution. For strong electrolytes, \( i \) represents the number of ions produced. - The relative lowering of vapour pressure is used to calculate the new vapour pressure of the solvent when a solute is added.
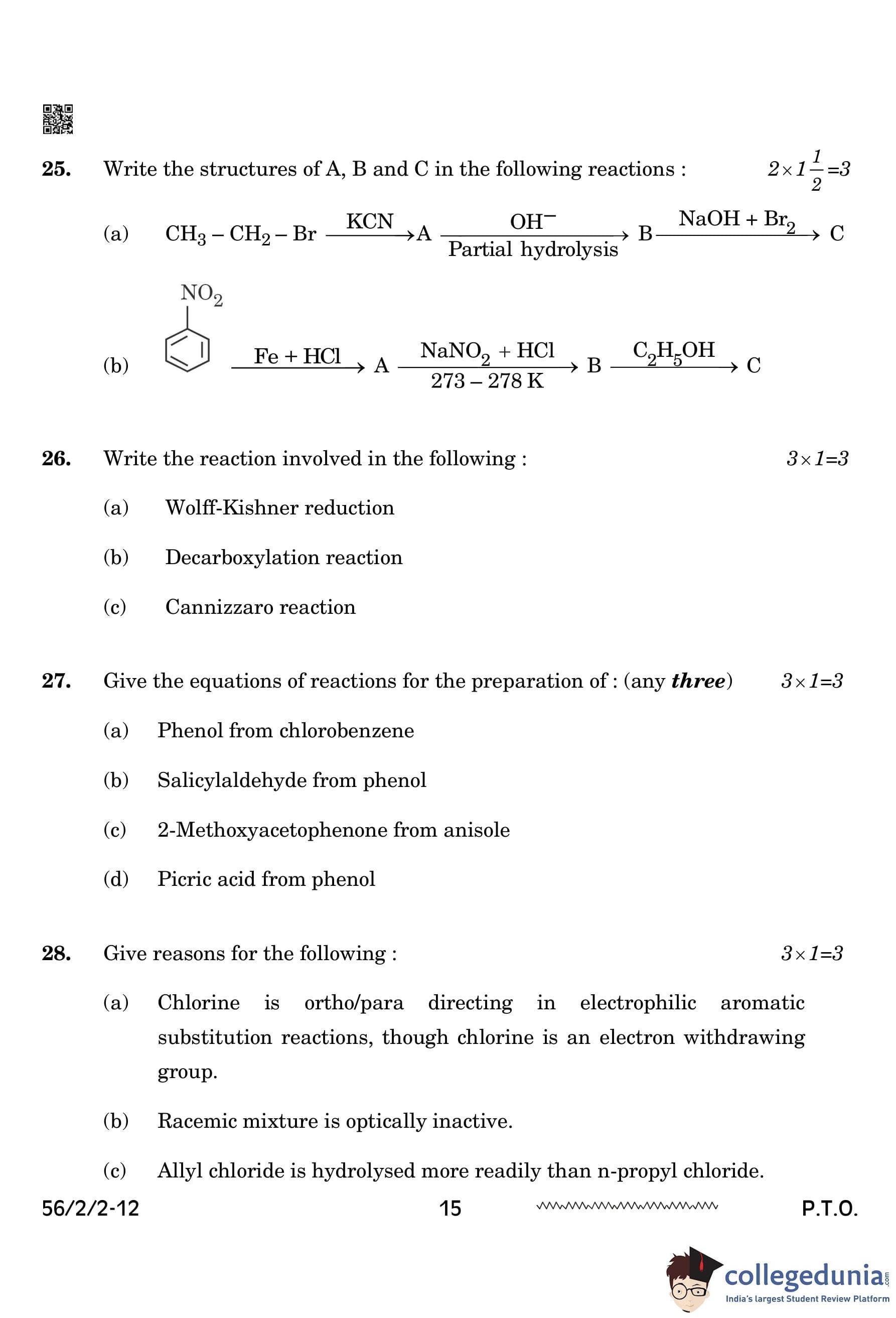
Write the structures of A, B, and C in the following reactions:
View Solution
(a) \[ A = CH_3CH_2CN \quad (Ethyl Cyanide) \] \[ B = CH_3CH_2CONH_2 \quad (Ethyl Amide) \] \[ C = CH_3CH_2NH_2 \quad (Ethyl Amine) \]
- Step 1: In this reaction, ethyl bromide (CH\(_3\)CH\(_2\)Br) reacts with KCN (potassium cyanide) in a nucleophilic substitution to form ethyl cyanide (A).
- Step 2: The ethyl cyanide (A) undergoes partial hydrolysis in the presence of OH\(^-\), converting the nitrile group to an amide to form ethyl amide (B).
- Step 3: Finally, the ethyl amide (B) reacts with NaOH + Br\(_2\) to form ethyl amine (C) through halogenation and basic hydrolysis.
(b)
- Step 1: Nitrobenzene reacts with Fe + HCl (reduction reaction) to form aniline (A).
- Step 2: The aniline (A) reacts with NaNO\(_2\) + HCl at 273-278 K to form diazonium salt (B).
- Step 3: Finally, the diazonium salt (B) undergoes coupling with phenol (C) to form phenol (C) in the presence of NaOH.
Quick Tip: - Ethyl cyanide and ethyl amide are intermediates in this nucleophilic substitution and hydrolysis sequence. - In diazotization reactions, the diazonium ion is formed by reacting amines with nitrous acid.
Write the reaction involved in the following:
(a) Wolff-Kishner reduction
View Solution
In the Wolff-Kishner reduction, a carbonyl compound (C=O) is treated with hydrazine (NH\(_2\)NH\(_2\)) and water to form a hydrazone intermediate (C=NH\(_2\)). The reaction is then heated in the presence of KOH and ethylene glycol, which removes the nitrogen as N\(_2\), resulting in the reduction of the carbonyl group to a methylene group (CH\(_2\)).
(b) Decarboxylation reaction
% Solution
Solution:
In the Decarboxylation reaction, sodium salts of aromatic carboxylic acids (Ar/RCOONa) undergo decarboxylation when treated with sodium hydroxide (NaOH) and calcium oxide (CaO) at high temperature (\(\Delta\)). This results in the formation of a hydrocarbon (Ar-H/R-H) and sodium carbonate (Na\(_2\)CO\(_3\)).
\[ Ar/RCOONa + NaOH \xrightarrow{CaO, \, \Delta} \, Ar-H/R-H + Na_2CO_3 \]
(c) Cannizzaro reaction
% Solution
Solution:
In the Cannizzaro reaction, formaldehyde (HCHO), in the presence of concentrated sodium hydroxide (NaOH) and heat (\(\Delta\)), undergoes disproportionation. This reaction involves two molecules of formaldehyde. One molecule is reduced to methanol (CH\(_3\)OH), while the other is oxidized to formate anion (HCOO\(^-\)), which then combines with a sodium ion (Na\(^+\)).
\[ 2HCHO \xrightarrow{Conc. NaOH, \, \Delta} \, HCOO^{-}Na^{+} + CH_3OH \] Quick Tip: - Wolff-Kishner reduction is a key method for reducing carbonyl groups to methylene groups (CH\(_2\)). - The decarboxylation reaction is widely used to remove carboxyl groups from aromatic compounds. - The Cannizzaro reaction is a redox reaction that occurs in the absence of an aldehyde with no hydrogen atom attached to the carbonyl group.
Give the equations of reactions for the preparation of: (any three)
(a) Phenol from chlorobenzene
View Solution
The Wurtz-Fittig reaction is used here. Chlorobenzene (C\(_6\)H\(_5\)Cl) undergoes nucleophilic substitution with NaOH under high temperature and pressure (623K, 300 atm), followed by acidic hydrolysis, to form phenol.
(b) Salicyaldehyde from phenol
% Solution
Solution:
Phenol (C\(_6\)H\(_5\)OH) undergoes haloform reaction with CHCl\(_3\) and NaOH to produce salicylic acid intermediate, which then undergoes acid hydrolysis to yield salicyaldehyde.
(c) 2-Methoxyacetophenone from anisole
% Solution
Solution:
Anisole (C\(_6\)H\(_5\)OCH\(_3\)) reacts with acetyl chloride (CH\(_3\)COCl) in the presence of anhydrous AlCl\(_3\) to form 2-methoxyacetophenone.
(d) Picric acid from phenol
% Solution
Solution:
Phenol is nitrated with concentrated nitric acid (HNO\(_3\)) to produce picric acid (2,4,6-trinitrophenol).
Quick Tip: - Phenol preparation via Wurtz-Fittig reaction involves nucleophilic substitution and hydrolysis. - Salicyaldehyde is obtained by the haloform reaction, a method of introducing an aldehyde group. - Acetylation of anisole forms 2-methoxyacetophenone through electrophilic substitution with acetyl chloride. - Nitration of phenol using conc. HNO\(_3\) introduces nitro groups and leads to picric acid.
Give reasons for the following:
(a) Chlorine is ortho/para directing in electrophilic aromatic substitution reactions, though chlorine is an electron withdrawing group.
View Solution
Chlorine is ortho/para directing in electrophilic aromatic substitution reactions
Although chlorine is an electron-withdrawing group through its inductive effect, it acts as an electron-donating group through resonance by donating lone pairs on chlorine to stabilize the intermediate carbocation. This effect is more pronounced at the ortho and para positions relative to the chlorine atom.
(b) Racemic mixture is optically inactive.
% Solution
Solution:
Racemic mixture is optically inactive
A racemic mixture contains two enantiomers in equal proportions. Enantiomers are non-superimposable mirror images of each other, and in a racemic mixture, the optical activities of these enantiomers cancel each other out, leading to zero optical rotation.
(c) Allyl chloride is hydrolysed more readily than n-propyl chloride.
% Solution
Solution:
Allyl chloride is hydrolysed more readily than n-propyl chloride
The allyl carbocation is stabilized by resonance with the adjacent double bond, making the reaction proceed more easily. In contrast, the n-propyl carbocation does not have this resonance stabilization, making it less stable and slower to undergo hydrolysis. Quick Tip: - Chlorine is ortho/para directing because of the stabilization of the carbocation through resonance. - Racemic mixtures result in optical inactivity due to the equal amounts of enantiomers canceling each other’s rotation. - Allyl chloride is more readily hydrolyzed than n-propyl chloride due to the resonance stabilization of the allyl carbocation.
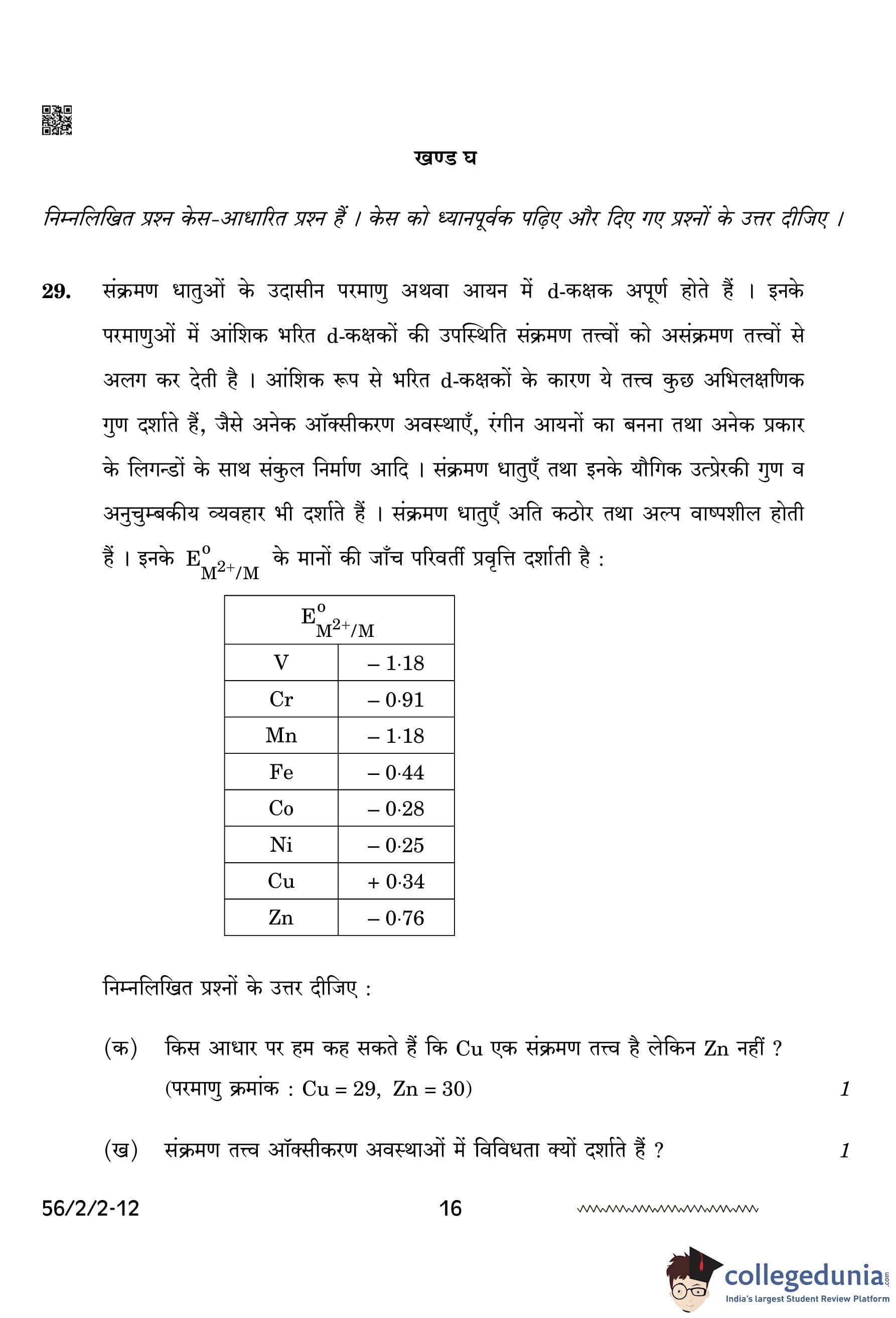
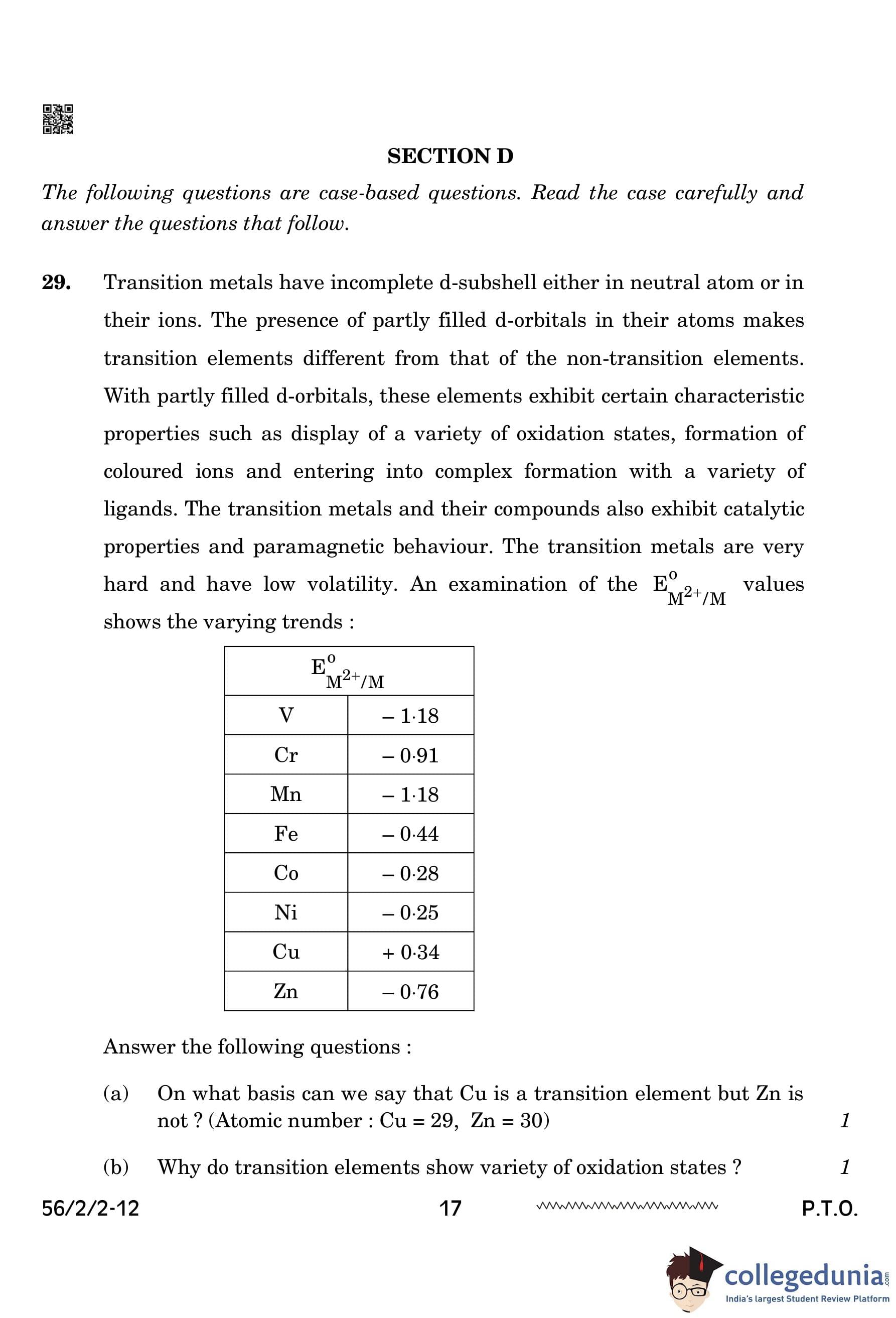
On what basis can we say that Cu is a transition element but Zn is not? (Atomic number: Cu = 29, Zn = 30)
View Solution
Cu is a transition element because it has an incomplete d-orbital in its +2 oxidation state, whereas Zn has a fully filled d-orbital in its ground state as well as in +2 oxidation state, making it a non-transition element. Transition elements must have at least one oxidation state with an incomplete d-orbital. Quick Tip: - Transition elements typically have an incomplete d-orbital in one or more oxidation states. - Non-transition elements like Zn have a full d-orbital in all of their oxidation states.
Why do transition elements show a variety of oxidation states?
View Solution
Transition elements show a variety of oxidation states because both (n-1)d and ns subshell electrons take part in bond formation due to their comparable energies. This allows for the formation of multiple oxidation states. The presence of unpaired electrons in d-orbitals also contributes to this variability. Quick Tip: - Transition metals can exhibit multiple oxidation states due to the participation of both d and s electrons in bonding. - The presence of unpaired electrons in the d-orbitals allows for flexibility in oxidation states.
Why do \(\text{E\(^0\)_{M^{2+/M \) values show irregular trends from Vanadium to Zinc?
View Solution
The irregular trends in \(\text{E\(^0\)_{M^{2+/M \) values from Vanadium to Zinc are due to irregular values of (\Delta
H\(_1\) + \Delta
H\(_2\)) and sublimation enthalpies. These values are influenced by factors like the ionization energies and the differences in the stability of the ions formed. Quick Tip: - Irregular trends in E\(^0\) values are often due to ionization energies, sublimation enthalpies, and other thermodynamic factors. - The transition elements show varying trends due to these complex thermodynamic effects.
How is the variability in oxidation states of transition metals different from that of non-transition elements?
View Solution
In transition metals, oxidation states vary by +1, whereas in non-transition metals, oxidation states differ by +2. This is due to the involvement of d-orbitals in transition metals, which allows for a greater variety of oxidation states. Quick Tip: - Transition metals have more flexibility in oxidation states because of the availability of d-orbitals. - Non-transition elements tend to show fewer oxidation states, typically differing by 2.
(i) Of the d\(^4\) species, Cr\(^{2+}\) is strongly reducing while Mn\(^{3+}\) is strongly oxidizing. Why? (Atomic number: Cr = 24, Mn = 25)
View Solution
Cr\(^{2+}\) is strongly reducing because it will be converted to Cr\(^{3+}\), which has a more stable half-filled t\(_2\)g configuration. On the other hand, Mn\(^{3+}\) is strongly oxidizing because it changes to Mn\(^{2+}\), which has a more stable half-filled d\(^5\) configuration. The stability of these configurations makes Cr\(^{2+}\) more likely to donate electrons, and Mn\(^{3+}\) more likely to accept electrons. Quick Tip: - Chromium in the +2 oxidation state is reducing, while Mn in the +3 oxidation state is oxidizing due to their electronic configurations. - The stability of half-filled d-orbitals drives these oxidation and reduction tendencies.
Complete the following ionic equation:
View Solution
The ionic equation is: \[ 2MnO_4^- + H_2O + I^- \rightarrow 2MnO_2 + 2OH^- + IO_3^- \] Quick Tip: - The reaction shows the reduction of MnO\(_4^-\) and the oxidation of I\(^-\). - The balance of charges and atoms is important when completing ionic equations.
What are reducing sugars?
View Solution
Reducing sugars are sugars that can reduce Tollens' reagent or Fehling’s solution due to the presence of a free aldehyde or ketone group. These groups are capable of donating electrons to reduce the reagents. Most monosaccharides like glucose, fructose, and galactose are reducing sugars. Disaccharides like lactose can also be reducing sugars if they have a free reducing end. Sucrose, however, is a non-reducing sugar since both of its anomeric carbons are involved in a glycosidic bond, preventing it from reducing reagents. Quick Tip: - Reducing sugars are sugars that reduce chemical reagents like Tollens' or Fehling’s solution. - The presence of free aldehyde or ketone groups in the sugar molecules allows them to act as reducing agents.
Classify the following into monosaccharides and disaccharides: Fructose, Sucrose, Lactose, Galactose
View Solution
- Monosaccharides: These are the simplest sugars and cannot be hydrolyzed into simpler sugars. Fructose and Galactose are both monosaccharides, as they consist of a single sugar unit.
- Disaccharides: These consist of two monosaccharides linked by a glycosidic bond. Sucrose and Lactose are disaccharides, formed by the combination of two monosaccharide units. Sucrose consists of glucose and fructose, while lactose consists of glucose and galactose. Quick Tip: - Monosaccharides like glucose, fructose, and galactose cannot be further hydrolyzed. - Disaccharides like sucrose and lactose can be broken down into two monosaccharides through hydrolysis.
Name the polysaccharide which is known as ‘animal starch’. Why is it called ‘animal starch’?
View Solution
The polysaccharide known as ‘animal starch’ is Glycogen. Glycogen is a branched polymer of glucose molecules, and it is the primary storage form of glucose in animals, particularly in the liver and muscles. It is similar to amylopectin, which is found in plants, but glycogen has more frequent branching. The name ‘animal starch’ reflects its role as the animal equivalent of plant starch, storing energy for later use. Quick Tip: - Glycogen is more highly branched than amylopectin, making it more readily accessible for quick energy release. - The structure of glycogen enables rapid mobilization of glucose when required by the body.
(i) Name the isomers of glucose which in the cyclic form differ only in the configuration of the –OH group at C – 1.
View Solution
The isomers of glucose that differ only in the configuration of the –OH group at C-1 in the cyclic form are \(\alpha\)-D-Glucose and \(\beta\)-D-Glucose. These are anomers, which are a type of stereoisomer that differ in the orientation of the substituent group (in this case, the –OH group) at the anomeric carbon (C-1). In \(\alpha\)-D-glucose, the –OH group at C-1 is on the opposite side of the ring relative to the CH2OH group, whereas in \(\beta\)-D-glucose, the –OH group at C-1 is on the same side as the CH2OH group. Quick Tip: - Anomers are cyclic sugars that differ in the configuration of the –OH group at the anomeric carbon. - \(\alpha\)-D-Glucose and \(\beta\)-D-Glucose are examples of anomers formed by glucose in its cyclic form.
Presence of which functional group was detected when glucose reacted with Br\(_2\) water?
View Solution
When glucose reacts with Br\(_2\) water, the presence of the aldehyde group (CHO) is detected. This is because the aldehyde group in glucose is capable of reducing bromine water, which leads to the oxidation of the aldehyde group. The reaction with Br\(_2\) water helps identify the presence of the aldehyde functional group in reducing sugars like glucose. Quick Tip: - The aldehyde group in glucose reacts with bromine water, indicating its reducing nature. - This reaction is a common test for the presence of aldehydes, which undergo oxidation to carboxylic acids.
The resistance of 0.05 M CH\(_3\)COOH solution is found to be 100 ohm. If the cell constant is 0.0354 cm\(^{-1}\), calculate the molar conductivity of the acetic acid solution.
View Solution
The molar conductivity (\(\Lambda_m\)) can be calculated using the formula: \[ \Lambda_m = \frac{k}{M} \times 1000 \]
Where:
- \(k\) is the conductivity,
- \(M\) is the molarity of the solution.
First, calculate the conductivity (\(k\)) using the given values: \[ k = \frac{1}{R} \times L/A = \frac{G}{R} \]
Where:
- \(R = 100 \, \Omega\),
- \(G = 0.0354 \, cm^{-1}\).
Thus: \[ k = \frac{1}{100} \times 0.0354 = 3.54 \times 10^{-4} \, \Omega^{-1} \, cm^{-1}. \]
Now, calculate \(\Lambda_m\) using the formula: \[ \Lambda_m = \frac{3.54 \times 10^{-4}}{0.05} \times 1000 = 7.08 \, S cm^2 \, mol^{-1}. \]
The molar conductivity of the acetic acid solution is \(7.08 \, S cm^2 \, mol^{-1}\). Quick Tip: - The molar conductivity is calculated by dividing the conductivity by the molarity and multiplying by 1000. - The cell constant \(G\) is used to calculate the conductivity of the solution from the resistance.
State Faraday's first law of electrolysis. How much charge in Faraday is required for the reduction of 1 mol of MnO\(_4^-\) to Mn\(^{2+}\)?
View Solution
Faraday's first law of electrolysis states that:
The amount of chemical reaction which occurs at any electrode during electrolysis is proportional to the quantity of electricity passed through the electrolyte.
The equation is: \[ m = ZIt \]
Where:
- \(m\) is the mass of the substance deposited,
- \(Z\) is the electrochemical equivalent,
- \(I\) is the current,
- \(t\) is the time.
For the reduction of 1 mole of MnO\(_4^-\) to Mn\(^{2+}\), the number of electrons required is 5 (because MnO\(_4^-\) is reduced to Mn\(^{2+}\) by gaining 5 electrons).
Thus, the charge required to reduce 1 mole of MnO\(_4^-\) to Mn\(^{2+}\) is given by: \[ Q = n \times F = 5 \times 96500 = 482500 \, Coulombs. \]
Therefore, the charge required for the reduction of 1 mole of MnO\(_4^-\) to Mn\(^{2+}\) is 482500 Coulombs. Quick Tip: - Faraday's law helps determine the amount of substance deposited during electrolysis based on the charge passed through the electrolyte. - The number of electrons involved in the reduction or oxidation process is key to calculating the total charge.
The conductivity of 0.0025 mol L\(^{-1}\) acetic acid is \(5.25 \times 10^{-5} \, S cm^{-1}\). Calculate its degree of dissociation if \(\Lambda_m^\circ\) for acetic acid is 390 S cm\(^2\) mol\(^{-1}\).
View Solution
The molar conductivity (\(\Lambda_m\)) is calculated as follows: \[ \Lambda_m = \frac{k}{M} \times 1000 \]
Where:
- \(k = 5.25 \times 10^{-5} \, S cm^{-1}\) (given conductivity),
- \(M = 0.0025 \, mol L^{-1}\) (molarity).
Thus, the molar conductivity is: \[ \Lambda_m = \frac{5.25 \times 10^{-5}}{0.0025} \times 1000 = 21 \, S cm^2 \, mol^{-1}. \]
The degree of dissociation (\(\alpha\)) is calculated by the formula: \[ \alpha = \frac{\Lambda_m}{\Lambda_m^\circ} = \frac{21}{390} = 0.053. \]
Thus, the degree of dissociation is 0.053. Quick Tip: - The degree of dissociation is the ratio of the observed molar conductivity to the theoretical molar conductivity at complete dissociation. - Molar conductivity depends on both concentration and the dissociation degree of the compound.
Write the anode, cathode, and overall reaction of lead storage battery.
View Solution
In a lead storage battery, the reactions at the anode, cathode, and overall reaction are as follows:
- Anode (oxidation): \[ Pb + SO_4^{2-} \rightarrow PbSO_4 + 2e^- \]
- Cathode (reduction): \[ PbO_2 + SO_4^{2-} + 4H^+ + 2e^- \rightarrow PbSO_4 + 2H_2O \]
- Overall reaction: \[ Pb + PbO_2 + 2H_2SO_4 \rightarrow 2PbSO_4 + 2H_2O \]
This is the reaction that takes place during the discharge of a lead storage battery. Quick Tip: - In a lead storage battery, lead and lead dioxide act as the electrodes, with sulfuric acid serving as the electrolyte. - The overall reaction involves the conversion of lead and lead dioxide into lead sulfate.
How is the crystal field splitting energy for octahedral complex (\(\Delta_o\)) related to that of tetrahedral complex (\(\Delta_t\))?
View Solution
The crystal field splitting energy for octahedral complex (\(\Delta_o\)) is related to that of the tetrahedral complex (\(\Delta_t\)) by the following equation: \[ \Delta_t = \left(\frac{4}{9}\right) \Delta_o \]
This relation arises due to the difference in the ligand field splitting between the two geometries. Quick Tip: - Octahedral complexes experience a larger splitting of d-orbitals compared to tetrahedral complexes. - The ratio of \(\Delta_t\) to \(\Delta_o\) is derived based on their geometry and ligand interactions.
Write the IUPAC name of the following complex: [PtCl\(_2\)(en)\(_2\)](NO\(_3\))\(_2\)
View Solution
The IUPAC name of the complex is Dichloridobis(ethane-1, 2-diamine)platinum(IV) nitrate.
Here, "en" stands for ethane-1,2-diamine, and platinum is in the +4 oxidation state. Quick Tip: - The IUPAC name involves naming ligands in alphabetical order before the metal, followed by its oxidation state. - "en" is the abbreviation for ethane-1,2-diamine, a bidentate ligand.
Write the geometry and magnetic behaviour of the complex [Ni(CO)\(_4\)] on the basis of Valency Bond Theory (VBT).
View Solution
The geometry of [Ni(CO)\(_4\)] is tetrahedral. According to Valency Bond Theory (VBT), the metal ion (Ni) undergoes sp\(^3\) hybridization to form four bonds with the CO ligands. The complex is diamagnetic as all the electrons in CO ligands are paired. Quick Tip: - In tetrahedral complexes, the central metal atom undergoes sp\(^3\) hybridization. - Diamagnetic behavior is observed when all electrons are paired, and there are no unpaired electrons in the system.
What type of isomerism is shown by the complex [Co(NH\(_3\))\(_6\)][Cr(CN)\(_6\)]?
View Solution
The complex [Co(NH\(_3\))\(_6\)][Cr(CN)\(_6\)] shows coordination isomerism. In this case, the ligands are interchangeable between the metal ions, leading to the possibility of different coordination compounds. Quick Tip: - Coordination isomerism occurs when ligands can be exchanged between the metal ions in a complex. - This type of isomerism is possible in complexes where there are two different metal ions with different ligands.
For the coordination compound on the basis of crystal field theory, write the electronic configuration for d\(^4\) ion if \(\Delta_o\) \(<\) P. Is the coordination compound a high spin or low spin complex?
View Solution
For a d\(^4\) ion with \(\Delta_o\) \(<\) P, the electronic configuration is \(t_{2g}^3 e_g^1\), which corresponds to a high spin complex. This is because the pairing energy is higher than the crystal field splitting energy, causing electrons to occupy higher energy orbitals rather than pair up. Quick Tip: - In high spin complexes, the electrons prefer to occupy higher energy orbitals to minimize pairing. - Low spin complexes occur when \(\Delta_o\) > P, leading to pairing of electrons.
Out of [Co(NH\(_3\))\(_6\)]\(^{3+}\) and [Co(NH\(_3\))\(_4\)Cl\(_2\)]\(^+\), which complex is heteroleptic and why?
View Solution
The complex [Co(NH\(_3\))\(_4\)Cl\(_2\)]\(^+\) is heteroleptic because it contains two different types of ligands (NH\(_3\) and Cl\(^-\)). In contrast, [Co(NH\(_3\))\(_6\)]\(^{3+}\) is homoleptic as it contains only one type of ligand (NH\(_3\)). Quick Tip: - Heteroleptic complexes contain more than one type of ligand. - Homoleptic complexes contain only one type of ligand.
Draw the structures of optical isomers of [PtCl\(_2\)(en)\(_2\)]\(^{2+}\).
View Solution
The optical isomers of [PtCl\(_2\)(en)\(_2\)]\(^{2+}\) are dextrorotatory (d) and levorotatory (l) isomers. These isomers are mirror images of each other and cannot be superimposed. They are non-superimposable, making them optical isomers.
Quick Tip: - Optical isomers are non-superimposable mirror images. - They rotate plane-polarized light in opposite directions.
Account for the following:
Oxidation of aldehydes is easier as compared to ketones.
The alpha (\(\alpha\)) hydrogen atoms of aldehydes are acidic in nature.
View Solution
Oxidation of aldehydes is easier because it involves the cleavage of the C-H bond, which is weaker than the C-C bond found in ketones. This makes aldehydes more susceptible to oxidation.
The alpha (\(\alpha\)) hydrogen atoms of aldehydes are acidic due to the electron-withdrawing nature of the carbonyl group. This effect stabilizes the conjugate base through resonance, making the alpha hydrogens more acidic. Quick Tip: - The C-H bond in aldehydes is weaker than the C-C bond in ketones, making aldehydes easier to oxidize. - The electron-withdrawing carbonyl group in aldehydes enhances the acidity of the alpha hydrogens.
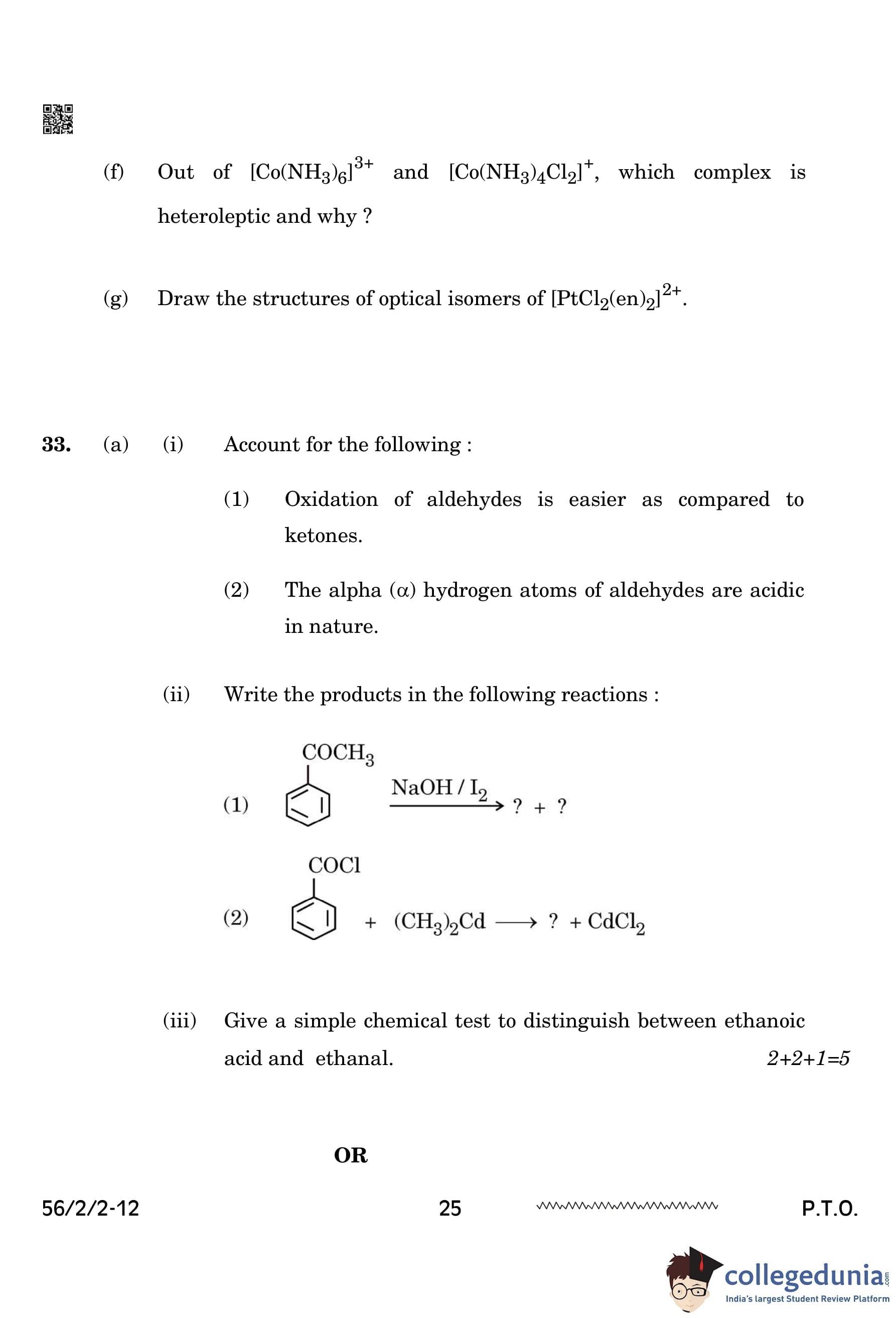
Write the products in the following reactions:
View Solution
The reaction of acetophenone (C\(_6\)H\(_5\)COCH\(_3\)) with NaOH and I\(_2\) forms a yellow precipitate of iodoform (CHI\(_3\)) as follows:
\[ C_6H_5COCH_3 + NaOH/I_2 \rightarrow C_6H_5COONa + CHI_3 \]
The reaction of benzoyl chloride (C\(_6\)H\(_5\)COCl) with dimethylcadmium ((CH\(_3\))\(_2\)Cd) results in the formation of a ketone (C\(_6\)H\(_5\)COCH\(_3\)) and cadmium chloride (CdCl\(_2\)) as follows:
\[ C_6H_5COCl + (CH_3)_2Cd \rightarrow C_6H_5COCH_3 + CdCl_2 \]
Quick Tip: - The reaction of aldehydes and methyl ketones with NaOH and I\(_2\) leads to the formation of iodoform (CHI\(_3\)). - The reaction with dimethylcadmium results in the formation of a ketone and the release of CdCl\(_2\).
Give a simple chemical test to distinguish between ethanoic acid and ethanal.
View Solution
A simple test to distinguish between ethanoic acid and ethanal is the iodoform test. When ethanal (acetaldehyde) is heated with NaOH and iodine (I\(_2\)), it forms a yellow precipitate of iodoform (CHI\(_3\)). This occurs because ethanal contains the structure –CHO (an aldehyde group) that can undergo oxidation and give a yellow iodoform precipitate. However, ethanoic acid (acetic acid), which contains a carboxyl group (–COOH), does not react in the same way. Therefore, ethanoic acid will not produce a yellow precipitate with NaOH and iodine, making this test a reliable way to differentiate the two. Quick Tip: - The iodoform test specifically identifies compounds with a methyl group directly attached to a carbonyl group, like ethanal or methyl ketones. - Ethanoic acid does not have a methyl group on its carbonyl group, so it does not give a yellow precipitate in the test.
Draw structure of the 2,4-dinitrophenylhydrazone of benzaldehyde.
View Solution
The structure of the 2,4-dinitrophenylhydrazone of benzaldehyde is shown below:
This compound is formed by the reaction of 2,4-dinitrophenylhydrazine with benzaldehyde. Quick Tip: - The formation of hydrazones is a common test for aldehydes and ketones. - The presence of nitro groups in the phenyl ring enhances the reactivity of the hydrazone derivative.
Arrange the following in increasing order of their reactivity towards HCN: \[ CH_3COCH_3, \, (CH_3)_3C - COCH_3, \, CH_3CHO \]
View Solution
The reactivity of these compounds towards HCN can be arranged in the following order: \[ (CH_3)_3C - COCH_3 < CH_3COCH_3 < CH_3CHO \]
Aldehydes are more reactive towards HCN than ketones due to the ease with which the carbonyl carbon in aldehydes is attacked. Quick Tip: - Aldehydes are generally more reactive than ketones because they have a less sterically hindered carbonyl group. - The presence of bulky groups in ketones like in tert-butyl ketone reduces the reactivity towards nucleophiles.
How can you convert phenyl magnesium bromide to benzoic acid?
View Solution
Phenyl magnesium bromide (C\(_6\)H\(_5\)MgBr) can be converted to benzoic acid by reacting it with carbon dioxide (CO\(_2\)) followed by hydrolysis. The reaction steps are: \[ C_6H_5MgBr + CO_2 \, (dry ether) \rightarrow C_6H_5COOMgBr \]
Then, hydrolyze the intermediate with water and acid: \[ C_6H_5COOMgBr + H_2O/H^+ \rightarrow C_6H_5COOH \]
This converts the Grignard reagent into benzoic acid. Quick Tip: - The reaction of a Grignard reagent with carbon dioxide and subsequent acid hydrolysis forms a carboxylic acid. - This reaction is known as the Grignard reaction for carboxylation.
Give a simple chemical test to distinguish between benzaldehyde and ethanal.
View Solution
On heating with NaOH and I\(_2\), ethanal gives a yellow precipitate of iodoform (CHI\(_3\)), whereas benzaldehyde does not react. This reaction is known as the iodoform test, which is specific for aldehydes with a methyl group on the carbonyl carbon. Quick Tip: - The iodoform test distinguishes aldehydes with a methyl group next to the carbonyl group, like ethanal. - Benzaldehyde does not react with NaOH and I\(_2\), making it a useful distinguishing feature.
Write the main product in the following reaction:
View Solution
The main product of the reaction is the reduction of the ester to a primary alcohol. Sodium borohydride (NaBH\(_4\)) reduces the ester group to an alcohol. The product after hydrolysis is 3-phenylpropanol.
The ester is reduced to the corresponding alcohol (3-phenylpropanol), and the acetic acid is formed as a by-product after hydrolysis. Quick Tip: - Sodium borohydride (NaBH\(_4\)) is a selective reducing agent that reduces esters to alcohols. - Hydrolysis of the intermediate gives the final alcohol and carboxylic acid.




















Comments